Notice regarding Section 508 of the Workforce Investment Act of 1998. Section 508 of the Workforce Investment Act of 1998 requires all United States Federal Agencies with websites to make them accessible to individuals with disabilities. At this time, the MPEP files below do not meet all standards for web accessibility. Until changes can be made to make them fully accessible to individuals with disabilities, the USPTO is providing access assistance via telephone. MPEP Interim Accessibility Contact: 571-272-8813.
1823.02 Nucleotide and/or Amino Acid Sequence Listings, and Tables Related to Sequence Listings [R-5] - 1800 Patent Cooperation Treaty
1823.02 Nucleotide and/or Amino Acid Sequence Listings, and Tables Related to Sequence Listings [R-5]
PCT RULE 5
The Description
*****
PCT RULE 5.2.
Nucleotide and/or Amino Acid Sequence Disclosure
(a) Where the international application contains disclosure of one or more nucleotide and/or amino acid sequences, the description shall contain a sequence listing complying with the standard prescribed by the Administrative Instructions and presented as a separate part of the description in accordance with that standard.
(b) Where the sequence listing part of the description contains any free text as defined in the standard provided for in the Administrative Instructions, that free text shall also appear in the main part of the description in the language thereof.
PCT RULE 13ter
Nucleotide and/or Amino Acid Sequence Listings
PCT RULE 13ter.1.
Procedure Before the International Searching Authority
(a) Where the international application contains disclosure of one or more nucleotide and/or amino acid sequences, the International Searching Authority may invite the applicant to furnish to it, for the purposes of the international search, a sequence listing in electronic form complying with the standard provided for in the Administrative Instructions, unless such listing in electronic form is already available to it in a form and manner acceptable to it, and to pay to it, where applicable, the late furnishing fee referred to paragraph (c), within a time limit fixed in the invitation:
(b) Where at least part of the international application is filed on paper and the International Searching Authority finds that the description does not comply with Rule 5.2(a), it may invite the applicant to furnish, for the purposes of the international search, a sequence listing in paper form complying with the standard provided for in the Administrative Instructions, unless such listing in paper form is already available to it in a form and manner acceptable to it, whether or not the furnishing of a sequence listing in electronic form is invited under paragraph (a), and to pay, where applicable, the late furnishing fee referred to in paragraph (c), within a time limit fixed in the invitation.
(c) The furnishing of a sequence listing in response to an invitation under paragraph (a) or (b) may be subjected by the International Searching Authority to the payment to it, for its own benefit, of a late furnishing fee whose amount shall be determined by the International Searching Authority but shall not exceed 25% of the international filing fee referred to in item 1 of the Schedule of Fees, not taking into account any fee for each sheet of the international application in excess of 30 sheets, provided that a late furnishing fee may be required under either paragraph (a) or (b) but not both.
(d) If the applicant does not, within the time limit fixed in the invitation under paragraph (a) or (b), furnish the required sequence listing and pay any required late furnishing fee, the International Searching Authority shall only be required to search the international application to the extent that a meaningful search can be carried out without the sequence listing.
(e) Any sequence listing not contained in the international application as filed, whether furnished in response to an invitation under paragraph (a) or (b) or otherwise, shall not form part of the international application, but this paragraph shall not prevent the applicant from amending the description in relation to a sequence listing pursuant to Article 34(2)(b).
(f) Where the International Searching Authority finds that the description does not comply with Rule 5.2(b), it shall invite the applicant to submit the required correction. Rule 26.4 shall apply mutatis mutandis to any correction offered by the applicant. The International Searching Authority shall transmit the correction to the receiving Office and to the International Bureau.
PCT RULE 13ter.2.
Procedure Before the International Preliminary Examining Authority
Rule 13ter.1 shall apply mutatis mutandis to the procedure before the International Preliminary Examining Authority.
PCT RULE 13ter.3.
Sequence Listing for Designated Office
No designated Office shall require the applicant to furnish to it a sequence listing other than a sequence listing complying with the standard provided for in the Administrative Instructions.
PCT Administrative Instructions Section 208
Sequence Listings
Any nucleotide and/or amino acid sequence listing ("sequence listing"), whether on paper or in electronic form, filed as part of the international application, or furnished together with the international application or subsequently, shall comply with Annex C.
I. REQUIREMENTS FOR SEQUENCE LISTINGS
Where an international application discloses one or more nucleotide and/or amino acid sequences, the description must contain a sequence listing complying with the standard specified in the Administrative Instructions. The standard is set forth in detail in Annex C - Standard for the Presentation of Nucleotide and Amino Acid Sequence Listings in International Patent Applications Under the PCT. The standard allows the applicant to draw up a single sequence listing which is acceptable to all receiving Offices, International Searching and Preliminary Examining Authorities for the purposes of the international phase, and to all designated and elected Offices for the purposes of the national phase. The International Searching Authority and the International Preliminary Examining Authority may, in some cases, invite the applicant to furnish a listing complying with that standard. The applicant may also be invited to furnish a listing in an electronic form provided for in the PCT Administrative Instructions. It is advisable for the applicant to submit a listing of the sequence in electronic form, if such a listing is required by the competent International Searching Authority or International Preliminary Examining Authority, together with the international application rather than to wait for an invitation by the International Searching Authority or International Preliminary Examining Authority.
The electronic form is not mandatory in international applications to be searched by the United States International Searching Authority or examined by the United States International Preliminary Examining Authority. However, if an electronic form of a sequence listing is not provided, a search or examination will be performed only to the extent possible in the absence of the electronic form. The U.S. sequence rules ( 37 CFR 1.821 - 1.825) and the PCT sequence requirements are substantively consistent. In this regard, full compliance with the requirements of the U.S. rules will ensure compliance with the applicable PCT requirements. For a detailed discussion of the U.S. sequence rules, see MPEP § 2420 - § 2421.04. **
II. QUALIFYING FOR POTENTIALLY REDUCED BASIC FEE BY FILING SEQUENCE LISTING AND/OR TABLES ON COMPACT DISC RATHER THAN ON PAPER
PCT Administrative Instructions Section 801
Filing of International Applications Containing Sequence Listings and/or Tables
(a) Pursuant to Rules 89bis and 89ter, where an international application contains disclosure of one or more nucleotide and/or amino acid sequence listings ("sequence listings"), the receiving Office may, if it is prepared to do so, accept that the sequence listing part of the description, as referred to in Rule 5.2(a) and/or any table related to the sequence listing(s) ("sequence listings and/or tables"), be filed, at the option of the applicant:
(i) only on an electronic medium in electronic form in accordance with Section 802; or
(ii) both on an electronic medium in electronic form and on paper in accordance with Section 802;
provided that the other elements of the international application are filed as otherwise provided for under the Regulations and these Instructions.(b) Any receiving Office which is prepared to accept the filing in electronic form of the sequence listings and/or tables under paragraph (a) shall notify the International Bureau accordingly. The notification shall specify the electronic media on which the receiving Office will accept such filings. The International Bureau shall promptly publish any such information in the Gazette.
(c) A receiving Office which has not made a notification under paragraph (b) may nevertheless decide in a particular case to accept an international application the sequence listings and/or tables of which are filed with it under paragraph (a).
(d) Where the sequence listings and/or tables are filed in electronic form under paragraph (a) but not on an electronic medium specified by the receiving Office under paragraph (b), that Office shall, under Article 14(1)(a)(v), invite the applicant to furnish to it replacement sequence listings and/or tables on an electronic medium specified under paragraph (b).
(e) Where an international application containing sequence listings and/or tables in electronic form is filed under paragraph (a) with a receiving Office which is not prepared, under paragraph (b) or (c), to accept such filings, Section 333(b) and (c) shall apply.
Part 8 of the Administrative Instructions became effective January 11, 2001. Under Administrative Instructions Section 801(a), applicants may file the nucleotide and/or amino acid sequence listing part of the description of an international application on an electronic medium in electronic form with certain receiving Offices. As of September 6, 2002, Part 8 of the Administrative Instructions was expanded to include tables related to sequence listings. At the present time, the United States Receiving Office (RO/US) has not notified the International Bureau (IB) under Administrative Instructions Section 801(b) that it will be generally accepting the filing of international applications under Administrative Instructions Section 801(a). The RO/US will, however, accept such applications in a particular case pursuant to Administrative Instructions Section 801(c), provided that applicant follows the Guidelines set forth below in subsection II. A.
PCT Administrative Instructions Section 803
Calculation of International Filing Fee for International Applications Containing Sequence Listings and/or Tables
Where sequence listings and/or tables are filed in electronic form under Section 801(a), the international filing fee payable in respect of that application shall include the following two components:
(i) a basic component calculated as provided in the Schedule of Fees in respect of all pages filed on paper (that is, all pages of the request, description (excluding sequence listings and/or tables if also filed on paper), claims, abstract and drawings), and
(ii) an additional component, in respect of sequence listings and/or tables, equal to 400 times the fee per sheet as referred to in item 1 of the Schedule of Fees, regardless of the actual length of the sequence listings and/or tables filed in electronic form and regardless of the fact that sequence listings and/or tables may have been filed both on paper and in electronic form.
Applicants will usually achieve a significant fee savings by filing international applications under Administrative Instructions Section 801(a) in situations where the sequence listings and/or tables consume over four hundred (400) combined pages. The potentially reduced international filing fee described in Administrative Instructions Section 803 is available to applications filed pursuant to the Guidelines below. Applicants who do not wish to file under Administrative Instructions Section 801(a) may submit the sequence listing part and any related tables under conventional filing procedures but will not be eligible for the potentially reduced international filing fee described in Administrative Instructions Section 803.
When filing an international application under Administrative Instructions Section 801(a) in the RO/US, applicant should not submit a paper copy of the Sequence Listing part and/or tables. If both a sequence listing part and a tables part are filed under Administrative Instructions Section 801(a), the sequence listing part and the tables part must not be filed on the same electronic medium. With specific regard to tables, only tables which are related to sequence listings, as referred to in PCT Rule 5.2(a), are covered under Part 8 of the Administrative Instructions. Currently, other types of table data may not be filed on electronic media.
A. Guidelines on Qualifying for Potentially Reduced International Filing Fee Under PCT Administrative Instructions Section 803
1. What To Submit
The applicant is required to submit a complete copy of the international application, wherein the sequence listing part and/or tables part of the application is submitted on electronic media rather than on paper. The application is to be accompanied by a transmittal letter entitled "Compact Disc Transmittal Sheet For Submission Of Sequence Listing and/or Tables To the United States Receiving Office Under PCT Administrative Instructions - Part 8."
(a) Complete International Application With Sequence Listing Part and/or Tables Part on Electronic Media
Applicant shall submit a paper copy of the complete international application, with the exception that the sequence listing part and/or tables part is provided on electronic media rather than on paper. Four (4) copies of the sequence listing part and/or three (3) copies of the tables part are to be included with the application, each copy on an electronic medium or set of electronic media if additional capacity is needed. One copy of the sequence listing part, called the "computer readable form" (CRF) copy required by the Administrative Instructions (see Annex C of the Administrative Instructions, paragraphs 39-46), may be submitted on any acceptable medium under 37 CFR 1.824(c), although compact disc (CD) media is preferred. All other copies must be submitted only on CD media as specified below:
(1) CD-R
Type: 120mm Compact Disc Recordable
Specification: ISO 9660, 650MB; or
(2) CD-ROM
Type: ISO/IEC 10149:1995, 120mm Compact Disc Read Only Memory
Specification: ISO 9660, 650MB
Each electronic medium shall be enclosed in a hard protective case within a padded envelope. If a sequence listing file is included, the four (4) sequence listing part copies shall be labeled as follows:
(1) "COPY 1 - SEQUENCE LISTING PART"
(2) "COPY 2 - SEQUENCE LISTING PART"
(3) "COPY 3 - SEQUENCE LISTING PART"
(4) "CRF" If tables file(s) are included, the three (3) tables part copies shall be labeled as follows: (1) "COPY 1 - TABLES PART" (2) "COPY 2 - TABLES PART" (3) "COPY 3 - TABLES PART" Additionally, the labeling shall contain the following information:
(1) Name of Applicant
(2) Title of Invention
(3) Applicant's or Agent's File Reference Number
(4) Date of Recording
(5) Computer Operating System Used
(6) Name of the Competent Authority (i.e. the RO/US)
(7) Indication that the sequence listing part and/or tables part is being filed under Administrative Instructions Section 801(a)
(8) If the sequence listing file and/or tables file(s) consumes more than one CD, an indication such as "DISK 1/3", "DISK 2/3", and "DISK 3/3"
(9) For a CD containing tables, an indication such as "TABLES 1 to 450"
Examples of properly labeled electronic media appear below.

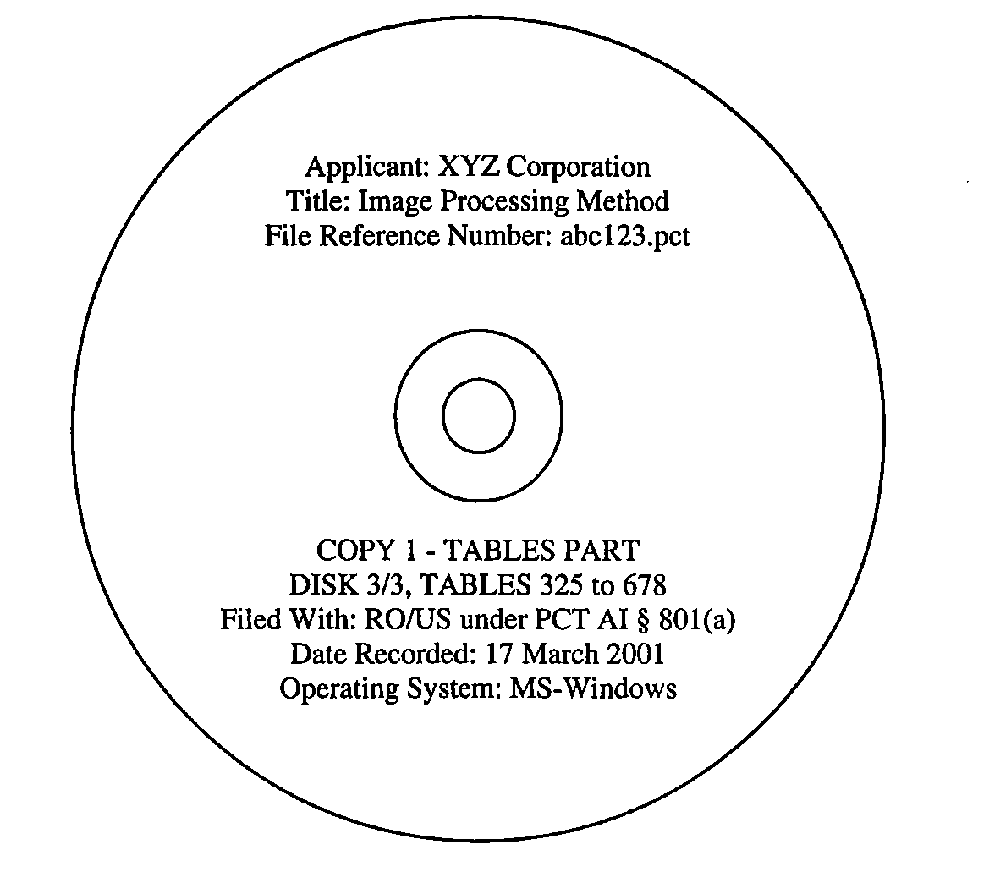
Important Notes:
The electronic medium itself must be neatly labeled with the required information. Labeling of the protective case is recommended, but not required. Sequence listings or tables submitted for correction, rectification, or amendment must satisfy the additional labeling requirements of Administrative Instructions Section 802(d).
Each CD shall contain either: (1) only a sequence listing part or (2) only a tables part. A sequence listing part and a tables part must not reside together on the same CD. Furthermore, each file in the tables part must have a file name which indicates the name of the table contained therein, e.g., "table-1.txt", "table-2.txt", etc. In addition, no programs or any explanatory files shall appear on any CD.
The sequence listing file and/or tables file(s) must be in compliance with the American Standard Code for Information Interchange (ASCII) and formatted in accordance with Administrative Instructions Annex C, paragraph 41 and Administrative Instructions Annex C-bis. No copy protection or encryption techniques are permitted. File compression is acceptable for the sequence listing part, so long as the compressed file is in a self-extracting format and uses the compression method described in Administrative Instructions Part 7, Annex F, Section 4.1.1. File Compression is not permitted for the tables part.
(b) Compact Disc Transmittal Sheet for Submission of Sequence Listing and/or Tables to the United States Receiving Office Under PCT Administrative Instructions - Part 8.
If applicant desires for an application to be accepted pursuant to Administrative Instructions Section 801(c), the application must be submitted with a document entitled "Compact Disc Transmittal Sheet For Submission Of Sequence Listing and/or Tables To The United States Receiving Office Under PCT Administrative Instructions - Part 8." This document is available as a PDF sheet that may be downloaded from http://www.uspto.gov/web/offices/pac/dapps/pct/part8translett.pdf. The PDF sheet includes the following information:
(1) Name of Applicant
(2) Applicant's or Agent's File Reference Number
(3) Title of Invention
(4) Name of Sequence Listing File and/or Tables File(s) (as per CD directory)
(5) Size of Sequence Listing File and/or Tables Files(s) (in bytes or kilobytes as per CD directory)
(6) Date of Sequence Listing File and/or Tables File(s) (as per CD directory)
(7) Statement that the four (4) submitted copies of the Sequence Listing Part and/or three (3) submitted copies of the Tables Part are identical
(8) Contact information
(a) Name of Contact
(b) Telephone Number
(c) Facsimile Number
(9) Signature of Applicant, Agent, or Common Representative
Important Note: The "Compact Disc Transmittal Sheet For Submission Of Sequence Listing and/or Tables To The United States Receiving Office Under PCT Administrative Instructions - Part 8" is separate and apart from any other transmittal letter. The Transmittal Sheet requirement cannot be satisfied by incorporating the above information into any other document. A sample copy of a "Compact Disc Transmittal Sheet for Submission of Sequence Listing To the United States Receiving Office Under PCT Administrative Instructions - Part 8" is reproduced on the following page.

2. Where To Submit
(a) United States Postal Service (Express Mail, Priority Mail, First Class Mail, etc.)
If deposited with the United States Postal Service, the entire international application, including all applicable items set forth in MPEP § 1823.02 paragraph II.A.1. above, should be addressed to:
Mail Stop PCT
Commissioner for Patents
P.O. Box 1450
Alexandria, VA 22313-1450
(b) Hand-Carried or by Private Delivery Service
If hand-carried or deposited with a private delivery service, the entire international application, including all applicable items set forth in MPEP § 1823.02 paragraph II.A.1. above, should be delivered to:
U.S. Patent and Trademark Office
Customer Service Window, Mail Stop PCT
Randolph Building
401 Dulany Street
Alexandria, VA 22314